The Cell living organisms.
Sunday, February 22, 2009
The cell is the structural and functional unit of all known living organisms. It is the smallest unit of an organism that is classified as living, and is often called the building block of life. Some organisms, such as most bacteria, are unicellular (consist of a single cell). Other organisms, such as humans, are multicellular. (Humans have an estimated 100 trillion or 1014 cells; a typical cell size is 10 µm; a typical cell mass is 1 nanogram.) The largest known cell is an unfertilized ostrich egg cell.
General principles
Each cell is at least somewhat self-contained and self-maintaining: it can take in nutrients, convert these nutrients into energy, carry out specialized functions, and reproduce as necessary. Each cell stores its own set of instructions for carrying out each of these activities.
All cells have several different abilities:
Reproduction by cell division: (binary fission/mitosis or meiosis).
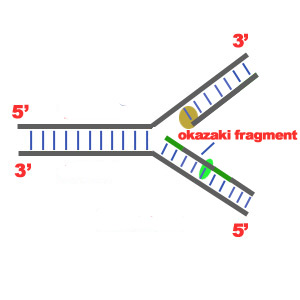
• Use of enzymes and other proteins coded for by DNA genes and made via messenger RNA intermediates and ribosomes.
• Metabolism, including taking in raw materials, building cell components, converting energy, molecules and releasing by-products. The functioning of a cell depends upon its ability to extract and use chemical energy stored in organic molecules. This energy is released and then used in metabolic pathways.
• Response to external and internal stimuli such as changes in temperature, pH or levels of nutrients.
• Cell contents are contained within a cell surface membrane that is made from a lipid bilayer with proteins embedded in it.
Some prokaryotic cells contain important internal membrane-bound compartments, but eukaryotic cells have a specialized set of internal membrane compartments.