RNA 5' Cap:
Monday, October 27, 2008
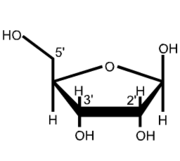
The 5' cap is a specially altered nucleotide end to the 5' end of precursor messenger RNA and some other primary RNA transcripts as found in eukaryotes and, as a special exception, caliciviruses such as norovirus. The process of 5' capping is vital to creating mature messenger RNA which is then able to undergo translation. Capping ensures the messenger RNA's stability while it undergoes translation in the process of protein synthesis, and is a highly regulated process which occurs in the cell nucleus. 5' cap structure Ribose structure showing the positions of the 2', 3' and 5' carbonsThe 5' cap is found on the 5' end of an mRNA molecule and consists of a guanine nucleotide connected to the mRNA via an unusual 5' to 5' triphosphate linkage. This guanosine is methylated on the 7 position directly after capping in vitro by a methyl transferase. It is referred to as a 7-methylguanosine cap, abbreviated m7G/ Further modifications include the possible methylation of the 2' hydroxy-groups of the first 3 ribose sugars of the 5' end of the mRNA. The methylation of both 2' hydroxy-groups is shown on the diagram